Biomedical and genetic engineers at Duke University and the Albert Einstein College of Medicine have developed a technique that naturally increases the presence of a light-sensitive molecule throughout the body. This change makes it possible to both improve deep tissue imaging in areas like the brain and expand the capabilities of light-based tools to control cellular behavior.
This technique enabled the researchers to induce the production of insulin and reduce blood glucose levels by nearly 60% in mouse models, illuminating a potential pathway for diabetes treatment. The work was published on July 14, 2025, in the journal Nature Communications.
Biliverdin is a light-sensitive biomolecule that is produced in large quantities in human and mammalian cells. Over the past decade, it has proven to be a useful target for both optogenetic and imaging tools because it can absorb near-infrared (NIR) light, which penetrates deeper into tissue than visible light.
Although biliverdin exists throughout the body, it is primarily found in blood-rich organs like the liver and spleen, leaving organs like the brain comparatively dark. But longtime collaborators Junjie Yao, an associate professor of biomedical engineering at Duke, and Vladislav Verkhusha, a professor of genetics at the Albert Einstein College of Medicine, developed a new technique to increase levels of biliverdin throughout the entire body.
“Injecting biliverdin directly into an animal can increase its levels to rise everywhere except the brain because it doesn’t efficiently cross the blood–brain barrier,” said Verkhusha, the co-director of the Gruss-Lipper Biophotonics Center. “But another way to increase the molecule is to remove the protein that eats it up.”
Researchers silenced biliverdin reductase-A, the enzyme that removes biliverdin by converting it to bilirubin, in mice. This caused levels of biliverdin to naturally rise throughout the animals, including in the brain. This rise made Verkhusha’s optogenetic proteins 25 times more effective at controlling gene expression throughout the mice using NIR light. They were also able to activate neurons 100 times higher in the brain.
As a proof of concept, the team used their optogenetic proteins to increase insulin production in a knockout mouse model of type 1 diabetes by making the liver produce insulin in response to NIR light. They observed a reduction in blood-glucose levels by nearly 60%, dropping it into a normal range and showing they could manage the condition noninvasively with NIR light.
“Although there have been limited studies in humans, animal studies indicate that there are no significant adverse health outcomes from silencing biliverdin reductase-A,” explained Verkhusha. “This suggests that we could effectively use optogenetics to naturally treat diabetes.”
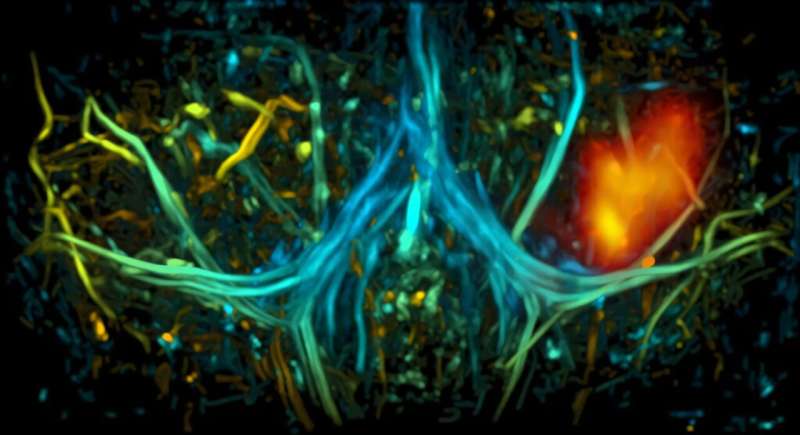
Yao and his team found similar success with deep tissue imaging using photoacoustic tomography (PAT), which involves shooting a laser beam of light into tissue and measuring the resulting ultrasonic wave.
“Most widely used optical imaging technologies aren’t able to penetrate deeper than a millimeter beneath the skin surface,” said Yao. “But if you bind NIR absorbing proteins to biliverdin, light can penetrate nearly three times deeper than standard probes, and image resolution can be two to three times clearer, allowing for detailed images of complex structures.”
This new approach allowed the team to see 7 millimeters into the brain, more than three times deeper than was possible without the biliverdin knockout model. The improved depth enabled the team to capture detailed images of neuronal activity in different areas of the brain through the animal’s scalp and skull. They were also able to illuminate blood flow and vascular structures, which they hope to use to better understand the relationship between neural activity and blood flow.
While this work provides researchers with an effective tool for imaging and optogenetic research, Verkhusha and Yao are both optimistic that it will set the stage for even more advancements.
“There are a lot of possibilities to explore as we move forward,” said Verkhusha. “We could continue exploring the use of optogenetic tools to make the liver generate therapeutic proteins like we did here with insulin.”
“For imaging, our next step could be to use this animal model to study more systematic and long-term diseases in different organ systems, like imaging the brain to study the effect of strokes or neurodegenerative diseases,” said Yao.
“Vlad and I have been collaborators for over a decade, and this is just another chapter in our ongoing work,” Yao said. “Our areas of research are different, but this partnership has been very complementary for both labs, and we’re excited to continue this fruitful work.”
More information:
Ludmila A. Kasatkina et al, Deep-tissue high-sensitivity multimodal imaging and optogenetic manipulation enabled by biliverdin reductase knockout, Nature Communications (2025). DOI: 10.1038/s41467-025-61532-4
Duke University
Citation:
Light-sensitive molecule boosts deep tissue imaging and cell control in mice (2025, August 1)
retrieved 1 August 2025
from https://medicalxpress.com/news/2025-08-sensitive-molecule-boosts-deep-tissue.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

