An estimated 50 million people worldwide have dementia, with Alzheimer’s disease—accounting for more than 70%—being the representative neurodegenerative brain disorder. A Korean research team has, for the first time, identified at the molecular level that tau and amyloid-β, the two key pathological proteins of Alzheimer’s disease, directly communicate to regulate toxicity.
This achievement is expected to provide new insights into the pathophysiology of Alzheimer’s disease, as well as important clues for discovering biomarkers for early diagnosis and developing therapeutics for neurodegenerative brain disorders. The research is published in the journal Nature Chemical Biology.
Professor Mi Hee Lim’s research team in the Department of Chemistry (Director of the Research Center for Metal–Neuroprotein Interactions) and multi-institutional collaborators have elucidated at the molecular level that the microtubule-binding domain of tau—one of the major pathological proteins of Alzheimer’s disease—directly interacts with amyloid-β (tau–amyloid-β communication), alters its aggregation pathway, and alleviates cellular toxicity.
Pathologically, Alzheimer’s disease is characterized by the accumulation of “neurofibrillary tangles” formed by aggregates of tau, a protein responsible for transporting nutrients and signaling molecules within neurons, and “amyloid plaques (senile plaques)” formed by clusters of amyloid-β fragments—abnormally cleaved from amyloid precursor protein, which is involved in brain development, intercellular signaling, and neuronal recovery—that aggregate in and around neuronal membranes in the brain.
Although tau and amyloid-β form pathological structures in spatially separated locations, it has been suggested that they may coexist inside and outside of cells and potentially interact. However, the molecular-level understanding of how their direct interaction affects the onset and progression of the disease has not been clearly revealed until now.
The joint research team found that among the structural repeats of tau protein that bind to microtubules (the intracellular transport system) inside neurons—K18, R1–R4, PHF6*, and PHF6—specifically K18, R2, and R3 bind with amyloid-β to form “tau–amyloid-β heterocomplexes.”
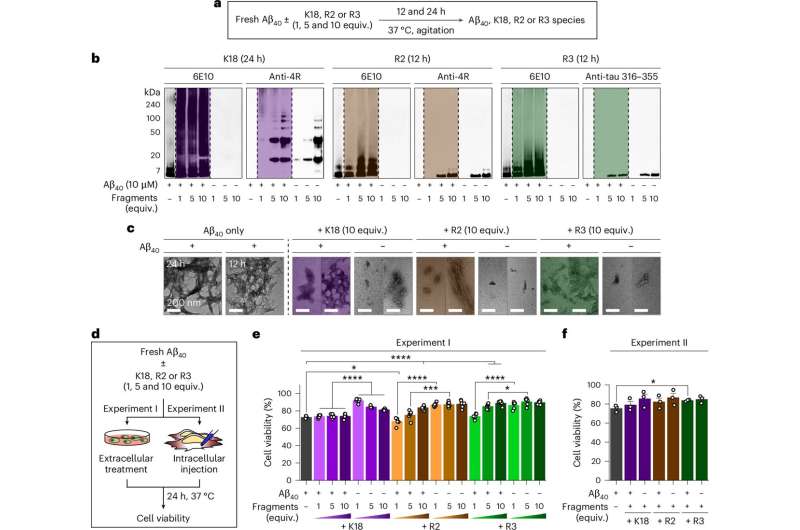
This process is significant because amyloid-β normally assembles into highly toxic, rigid fibers (amyloid fibrils), but when certain tau regions bind, amyloid-β shifts to an aggregation pathway that produces less toxic, less rigid aggregates.
Notably, these repeat regions of tau delay the nucleation stage (the initial step of amyloid aggregation linked to disease onset) and simultaneously alter the aggregation speed and structural form of amyloid-β associated with disease progression. As a result, the toxicity caused by amyloid-β was markedly reduced in both the intracellular and extracellular environments of the brain.
In this study, the team combined precise analytical techniques—including spectroscopy, mass spectrometry, isothermal titration calorimetry, and nuclear magnetic resonance—with cell-based toxicity assays to comprehensively analyze the structural, thermodynamic, and functional properties of tau–amyloid interactions.
The findings revealed that specific regions of tau’s microtubule-binding repeats possess both hydrophilic (water-attracting) and hydrophobic (water-repelling) characteristics, and when the balance of these two properties is optimized, tau binds more effectively to amyloid-β. In other words, the intrinsic properties of tau determine its binding affinity with amyloid-β, its modulation of aggregation pathways, and its ability to regulate toxicity.
Dr. Young-Ho Lee of KBSI stated, “This research has uncovered a new molecular mechanism for the onset and progression of dementia, an intractable neurodegenerative disease. In particular, multidisciplinary convergent research focused on molecular interactions and protein aggregation is expected to play a pivotal role in clarifying not only the cross-talk between Alzheimer’s and Parkinson’s diseases, but also the interconnections among various diseases such as dementia, diabetes, and cancer.”
Professor Mi Hee Lim of KAIST added, “Tau protein does not merely contribute to pathological formation, but rather, through specific microtubule-binding repeat structures, it exerts a molecular function that actively mitigates amyloid-β aggregation and toxicity. This provides a new turning point in the pathological understanding of Alzheimer’s disease. The significance of this study lies in identifying new molecular motifs that could serve as therapeutic targets not only for Alzheimer’s but also for a variety of protein aggregation-based neurodegenerative brain disorders.”
More information:
Mingeun Kim et al, Interactions with tau’s microtubule-binding repeats modulate amyloid-β aggregation and toxicity, Nature Chemical Biology (2025). DOI: 10.1038/s41589-025-01987-0
The Korea Advanced Institute of Science and Technology (KAIST)
Citation:
Communication between tau and amyloid-β proteins found to mitigate Alzheimer’s toxicity (2025, August 25)
retrieved 25 August 2025
from https://medicalxpress.com/news/2025-08-communication-tau-amyloid-proteins-mitigate.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

